

For gases the opposite is true, the volume of a gas can be measured in a gas burette, if care is taken to control the pressure. The volume of solids, particularly of powders, is often difficult to measure, which is why mass is the more usual measure. The use of graduated beakers and cylinders is not recommended as their indication of volume is mostly for decorative rather than quantitative purposes. For very small volumes precision syringes are available. The volume of a liquid is usually determined by calibrated glassware such as burettes and volumetric flasks. Both solids and liquids are easily quantified by weighing. Mass can be determined at a precision of < 0.2 mg on a routine basis with an analytical balance and more precise instruments exist. The measurement of mass does not require such restrictions. Unless otherwise stated, all the following measurements of volume are assumed to be at a standard state temperature and pressure (for example 25 degrees Celsius at 1 atmosphere or 101.325 kPa ). Volume-based measures for concentration are therefore not to be recommended for non-dilute solutions or problems where relatively large differences in temperature are encountered (e.g. This is why volumes are not necessarily completely additive when two liquids are added and mixed. In fact (partial) molar volume can even be a function of concentration itself.

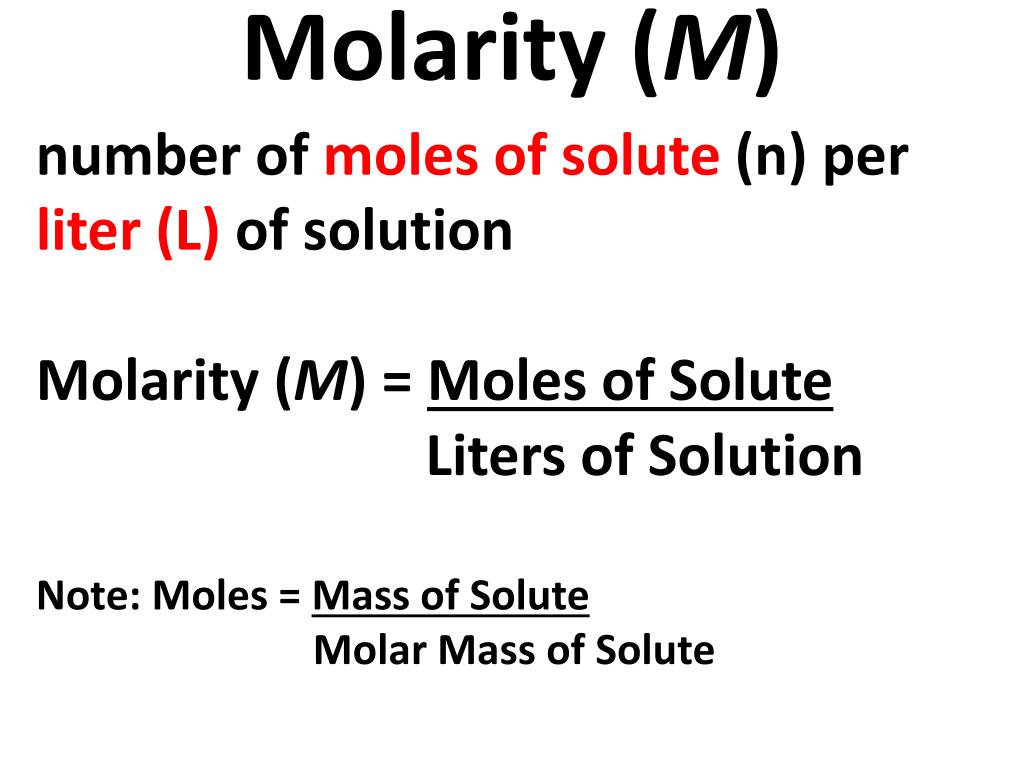
Some units of concentration -particularly the most popular one (molarity)- require knowledge of a substance's volume, which -in contrast to mass- is the variable depending on ambient temperature and pressure. At times this information may not be available, particularly if the temperature varies. Depending on what they are based on it is not always trivial to convert one measure to the other, because knowledge of the density might be needed to do so. They are based on mass or volume or both. There are a number of different ways to quantitatively express concentration the most common are listed below.
For example, a practical rule is that the more concentrated a chromatic solution is, the more intensely colored it is.įor scientific or technical applications, a qualitative account of concentration is almost never sufficient, therefore quantitative measures are needed to describe concentration. Those terms relate the amount of a substance in a mixture to the observable intensity of effects or properties caused by that substance. Often in informal, non-technical language, concentration is described in a qualitative way, through the use of adjectives such as "dilute" or "weak" for solutions of relatively low concentration and of others like "concentrated" or "strong" for solutions of relatively high concentration. The point of saturation depends on many variables such as ambient temperature and the precise chemical nature of the solvent and solute.Īnalytical concentration includes all the forms of that substance in the solution. Instead, phase separation will occur, leading to either coexisting phases or a suspension. If additional solute is added to a saturated solution, it will not dissolve (except in certain circumstances, when supersaturation may occur).

At this point, the solution is said to be saturated. Unless two substances are fully miscible there exists a concentration at which no further solute will dissolve in a solution. By contrast, to dilute a solution, one must add more solvent, or reduce the amount of solute. To concentrate a solution, one must add more solute, or reduce the amount of solvent (for instance, by selective evaporation). This can apply to any sort of chemical mixture, but most frequently the concept is limited to homogeneous solutions, where it refers to the amount of solute in a substance. In chemistry, concentration is the measure of how much of a given substance there is mixed with another substance.


 0 kommentar(er)
0 kommentar(er)
